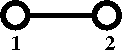Mayer f-function: Difference between revisions
Jump to navigation
Jump to search
Carl McBride (talk | contribs) No edit summary |
m (link to Boltzmann factor) |
||
| Line 7: | Line 7: | ||
* <math>T</math> is the [[temperature]]. | * <math>T</math> is the [[temperature]]. | ||
* <math>\Phi_{12}(r)</math> is the [[intermolecular pair potential]]. | * <math>\Phi_{12}(r)</math> is the [[intermolecular pair potential]]. | ||
In other words, the Mayer function is the [[Boltzmann factor]] of the interaction potential, | |||
minus one. | |||
[[Cluster diagrams | Diagrammatically]] the Mayer ''f''-function is written as | [[Cluster diagrams | Diagrammatically]] the Mayer ''f''-function is written as | ||
Revision as of 12:42, 22 February 2008
The Mayer f-function, or f-bond is defined as (Ref. 1 Chapter 13 Eq. 13.2):
- Failed to parse (Conversion error. Server ("https://wikimedia.org/api/rest_") reported: "Cannot get mml. Server problem."): {\displaystyle f_{12}=f({\mathbf {r} }_{12})=\exp \left(-{\frac {\Phi _{12}(r)}{k_{B}T}}\right)-1}
where
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle k_B} is the Boltzmann constant.
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T} is the temperature.
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Phi_{12}(r)} is the intermolecular pair potential.
In other words, the Mayer function is the Boltzmann factor of the interaction potential, minus one.
Diagrammatically the Mayer f-function is written as
Hard sphere model
For the hard sphere model the Mayer f-function becomes:
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle f_{12}= \left\{ \begin{array}{lll} -1 & ; & r_{12} \leq \sigma ~~({\rm overlap})\\ 0 & ; & r_{12} > \sigma ~~({\rm no~overlap})\end{array} \right. }
where Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \sigma} is the hard sphere diameter.
References
- Joseph Edward Mayer and Maria Goeppert Mayer "Statistical Mechanics" John Wiley and Sons (1940)
- Joseph E. Mayer "Contribution to Statistical Mechanics", Journal of Chemical Physics 10 pp. 629-643 (1942)