SPC model of water: Difference between revisions
Jump to navigation
Jump to search
Carl McBride (talk | contribs) m (Updated internal link) |
Carl McBride (talk | contribs) m (→Related models: Updated links) |
||
| (12 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
The '''simple point charge''' (SPC) model | |||
The '''simple point charge''' (SPC) model is an [[ | <ref>H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren and J. Hermans, in: Intermolecular Forces (B. Pullman, ed.), Reidel, Dordrecht (1981) p. 331 ISBN 902771326X</ref> | ||
a rigid isosceles triangle, having charges situated on each of the three atoms. | is an [[water models | empirical model of water]]. The molecule is modelled as | ||
[[Image: | a rigid isosceles triangle, having charges situated on each of the three atoms. As well as [[Coulomb's law |Coulombic interactions]], the molecules interact via long-range [[Lennard-Jones model | Lennard-Jones]] sites, situated on the oxygen atoms. The parameters are as follows: | ||
[[Image:Thee_site_water_model.png|center|400px]] | |||
{| border="1" | {| border="1" | ||
|- | |- | ||
| parameter || value | | parameter || value | ||
|- | |- | ||
| <math>\sigma</math> || <math> 3.166 | | <math>\sigma</math> || <math> 3.166</math>Å | ||
|- | |- | ||
| <math>\epsilon</math> || <math>0.650</math> kJ mol<sup>-1</sup> | | <math>\epsilon</math> || <math>0.650</math> kJ mol<sup>-1</sup> | ||
|- | |- | ||
| <math>r_\mathrm{OH}</math> || <math>1.000 | | <math>r_\mathrm{OH}</math> || <math>1.000</math>Å | ||
|- | |- | ||
| <math>\angle_\mathrm{HOH}</math> || <math>109.47^{\circ}</math> | | <math>\angle_\mathrm{HOH}</math> || <math>109.47^{\circ}</math> | ||
| Line 17: | Line 18: | ||
| <math>q_{\mathrm{O}}</math> || <math>-0.82 e</math> | | <math>q_{\mathrm{O}}</math> || <math>-0.82 e</math> | ||
|- | |- | ||
| <math>q_{\mathrm{H}}</math> || <math>q_{\mathrm{O}}/2</math> (charge neutrality | | <math>q_{\mathrm{H}}</math> || <math>|q_{\mathrm{O}}|/2</math> (charge neutrality) | ||
|} | |} | ||
The SPC model has a [[dipole moment]] of 2.27 D. | The SPC model has a [[dipole moment]] of 2.27 D. | ||
==Surface tension== | |||
The [[surface tension]] has been studied for the SPC model by Vega and Miguel. | |||
<ref>[http://dx.doi.org/10.1063/1.2715577 C. Vega and E. de Miguel "Surface tension of the most popular models of water by using the test-area simulation method", Journal of Chemical Physics '''126''' 154707 (2007)]</ref> | |||
==Related models== | |||
Over the years a number of variants of the SPC model have been published: | |||
*[[SPC/ε water model | SPC/ε]] | |||
*[[SPC/A]] | |||
*[[SPC/E]] | |||
*[[SPC/F]] | |||
*[[SPC/F2]] | |||
*[[SPC/FP]] | |||
*[[SPC/FQ]] | |||
*[[SPC/Fw]] | |||
*[[SPC/HW]] | |||
*[[SPC/L]] | |||
*[[SPCP]] | |||
*[[SPC-pol]] | |||
==References== | ==References== | ||
<references/> | |||
[[category: water]] | [[category: water]] | ||
[[category: models]] | [[category: models]] | ||
Latest revision as of 15:20, 9 February 2015
The simple point charge (SPC) model [1] is an empirical model of water. The molecule is modelled as a rigid isosceles triangle, having charges situated on each of the three atoms. As well as Coulombic interactions, the molecules interact via long-range Lennard-Jones sites, situated on the oxygen atoms. The parameters are as follows:
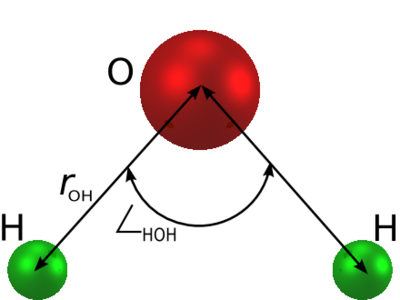
| parameter | value |
| Å | |
| kJ mol-1 | |
| Å | |
| (charge neutrality) |
The SPC model has a dipole moment of 2.27 D.
Surface tension[edit]
The surface tension has been studied for the SPC model by Vega and Miguel. [2]
Related models[edit]
Over the years a number of variants of the SPC model have been published:
References[edit]
- ↑ H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren and J. Hermans, in: Intermolecular Forces (B. Pullman, ed.), Reidel, Dordrecht (1981) p. 331 ISBN 902771326X
- ↑ C. Vega and E. de Miguel "Surface tension of the most popular models of water by using the test-area simulation method", Journal of Chemical Physics 126 154707 (2007)











